Availability of Psychotropic Substances
Ensuring availability of psychotropic substances for mental health and other licit purposes
Parties to the 1971 Convention on Psychotropic Substances, while expressing a determination to prevent and combat abuse of and trafficking in psychotropic substances, recognized that the use of these substances for medical and scientific purposes was indispensable and that their availability for such purposes should not be unduly restricted.
As such, the 1971 Convention provides a legal framework for the control of a number of important and indispensable medicines. Psychotropic substances are essential for the treatment and management of a wide range of medical conditions, in particular mental and neurological health conditions, such as anxiety, insomnia and epilepsy, and for the induction of anaesthesia in preoperative procedures.
The crucial importance of medicines containing psychotropic substance became even more evident with the COVID-19 pandemic, during which a number of substances were used for the treatment of patients in ICU units and for people who faced mental health difficulties as a result of the pandemic. A number of countries have suffered shortages in the supply of controlled psychotropics and the Board has been working with countries to ensure uninterrupted international trade during this emergency situation.
Despite the important role that internationally controlled psychotropic substances play in the medical environment, assessing their global, regional and national availability remains a challenge as neither comprehensive data at the national level nor well-established ways of assessing the appropriate level of use of psychotropic substances to meet demand exist.
Data received from a limited number of Governments indicate that while 80 per cent of people living with epilepsy live in low- and middle-income countries, the consumption of related psychotropic substances is concentrated in high-income countries. This reflects on the one hand a diversity in medical practice and related variations in prescription patterns, and on the other hand a lack of accurate data, both quantitative and qualitative, on the consumption of such substances. For further details, see the 2018 INCB special report entitled: Progress in Ensuring Adequate Access to Internationally Controlled Substances for Medical and Scientific Purposes
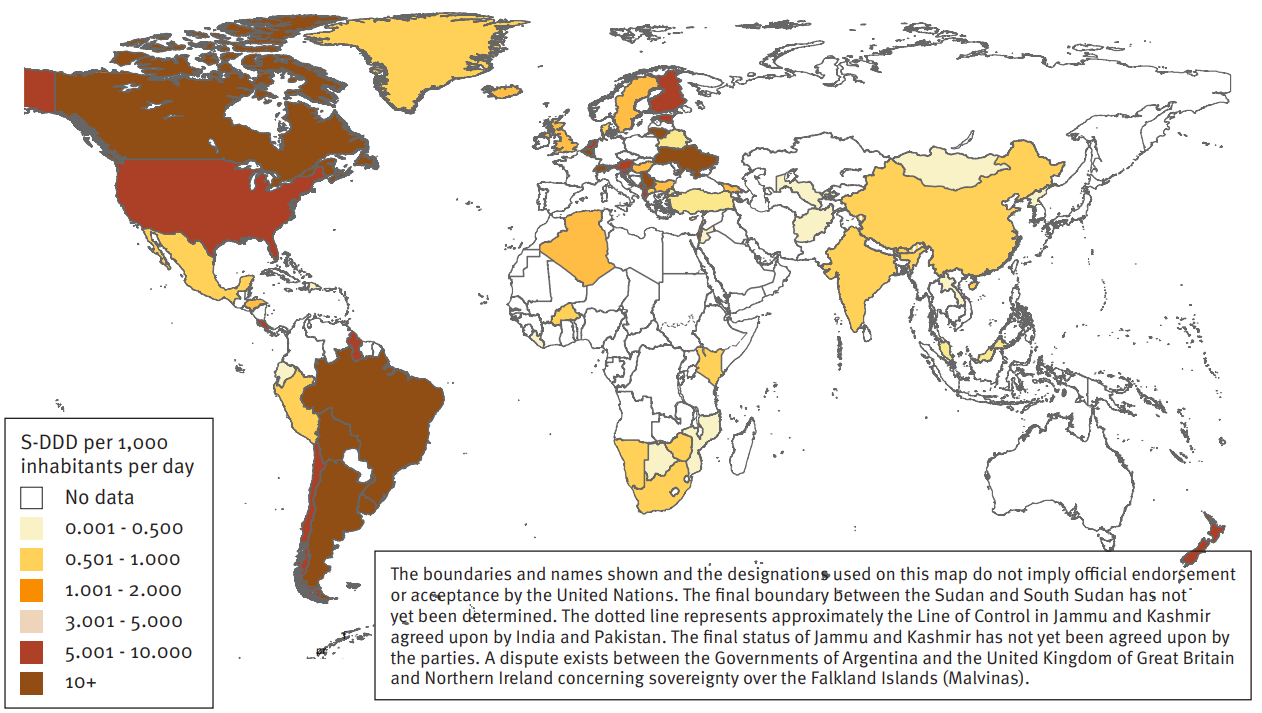
Map showing average consumption of selected anti-epileptics in S-DDD per 1,000 inhabitants per day for 2016
The Board has faced a challenge in monitoring and assessing the availability of psychotropic substances in many parts of the world, due to inconsistent or non-existent consumption data. Available information indicates that these substances may be almost inaccessible to some populations, and that resources allocated to addressing mental health disorders may have been inadequately and inequitably distributed.
In addition, the lack of data on the quantities of psychotropic substances consumed in many parts of the world remains the main challenge in providing appropriate assistance to countries where availability is low. Governments thus continue to be urged to assess their medical needs, measure their national consumption and submit such data to the INCB.
Measuring national consumption
The 1971 Convention does not provide a definition of consumption of psychotropic substances falling under its control regime, nor does it require State Parties to submit data on national consumption. The Commission on Narcotic Drugs adopted multiple resolutions aimed at increasing the Board's cooperation with Governments in gaining a clearer picture of the levels of consumption of psychotropic substances worldwide.
In its resolution 53/4 of March 2010, the Commission invited the Board to include in its annual report for 2010 information on the consumption of psychotropic substances. In its resolution 54/6 of March 2011, the Commission encouraged Member States to report data on the consumption of psychotropic substances for medical and scientific purposes to INCB, in order to enable the Board to analyse levels of consumption of psychotropic substances in an accurate manner and to promote their adequate availability.
Thus, parties to the 1971 Convention are encouraged to include in their annual statistical reports (using Form P) data on the consumption of psychotropic substances. For each psychotropic substance listed in the four schedules of the 1971 Convention, the reporting authority should indicate the quantity consumed during the year in question. For further information on how to fill out Form P, see the training material for competent national authorities.
To assist countries in their consumption data collection, the Board has compiled information on the most commonly used methodologies for the collection of data on consumption of psychotropic substances.
Compilation of Methodologies for Collecting Data on the Consumption of Psychotropic Substances
The Compilation is intended to serve as the starting point for the development of data collection methodologies by competent national authorities that do not report data on the consumption of psychotropic substances to INCB. It may also serve countries that have a data collection system in place to measure national consumption, but who wish to consult other methodologies available and used in other countries.
It also includes information on the mechanisms used by some competent national authorities to validate the data collected from their licensed operators, as well as details about common operational practices in the data collection process, and about new technologies shaping health information systems.
The Board invites countries to share information and/or other methodologies identified within their territories, to be included in the compilation.
Data analysis
The Board analyses consumption patterns and trends in all parts of the world, albeit with limited data it receives. This information is published in its technical report related to psychotropic substances.
Questions?
Tel: (43 - 1) 26060 - 4277
E-mail:
incb.psychotropics@un.org
