Scheduling procedure for drug precursors
This scheduling procedure allows to subject substances frequently used in illicit drug manufacture to international control by adding them to Table I or II of the 1988 Convention. The procedure typically takes several months and involves several stakeholders. The same procedure applies for the deletion of a substance from Table I or Table II, or for the transfer of a substance from one Table to another.
Procedure for adding chemicals to the Tables of the 1988 Convention
Who can initiate a scheduling procedure?
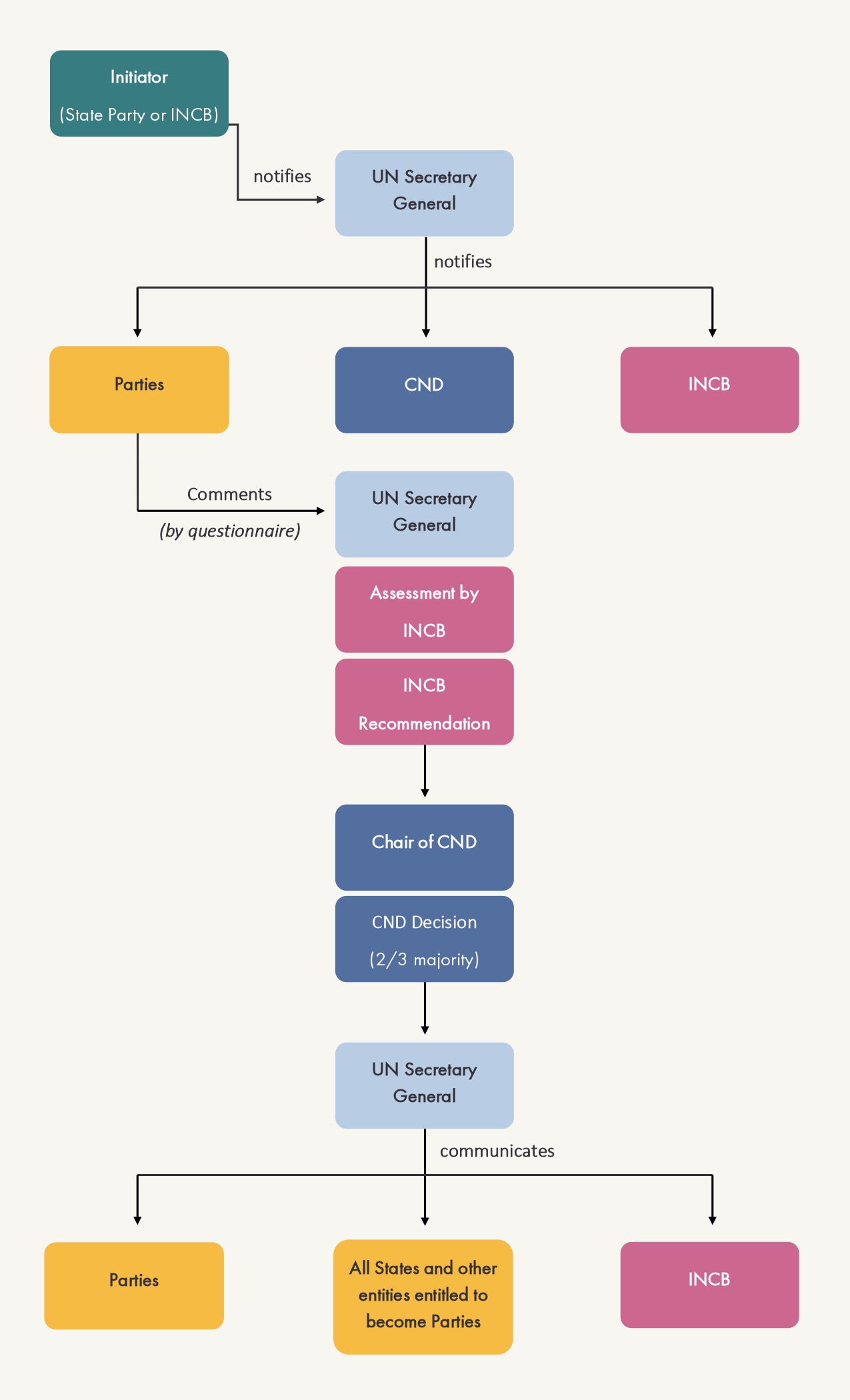
The scheduling procedure can be initiated by any State party to the Convention or by INCB. The initiator notifies the Secretary-General of the United Nations of the suggested scheduling change and provides them with supporting information. The Secretary-General then transmits the notification to the State parties, the Commission on Narcotic Drugs (CND) and INCB.
What is the States parties' role during and after the scheduling procedure?
States parties provide comments concerning the notification, typically via a questionnaire, as well as any supplementary information to contribute to the assessment of a substance that is being considered for scheduling.
After a scheduling decision becomes effective, State parties should enact national legislation to comply with the provisions of article 12 of the Convention. A number of tools have been made available by INCB to support Governments in their efforts.
Governments' obligations related to substances listed in the Tables of the 1988 Convention
What is the role of INCB?
INCB conducts the assessment and makes a scheduling recommendation to be voted on by the CND. Under article 12 paragraph 4, INCB examines information provided by the States parties and its decision shall reflect:
- The extent, importance and diversity of licit use;
- The possibility and ease of using alternate substances (both for licit and illicit purposes);
- The frequency of use in illicit drug manufacture;
- Whether volume and extent of illicit manufacture of the drug end product create serious public health or social problems, so as to warrant international action.
What happens during and after the CND vote?
INCB's scheduling recommendation is voted by the CND. A two-thirds majority (at least 36 Members of the CND) must vote in favor to take a decision to add a substance to, remove it from, or transfer it between the Tables of the 1988 Convention. The Secretary-General then communicates the decision to all States. Decisions enter into force 180 days after the date of such communication.
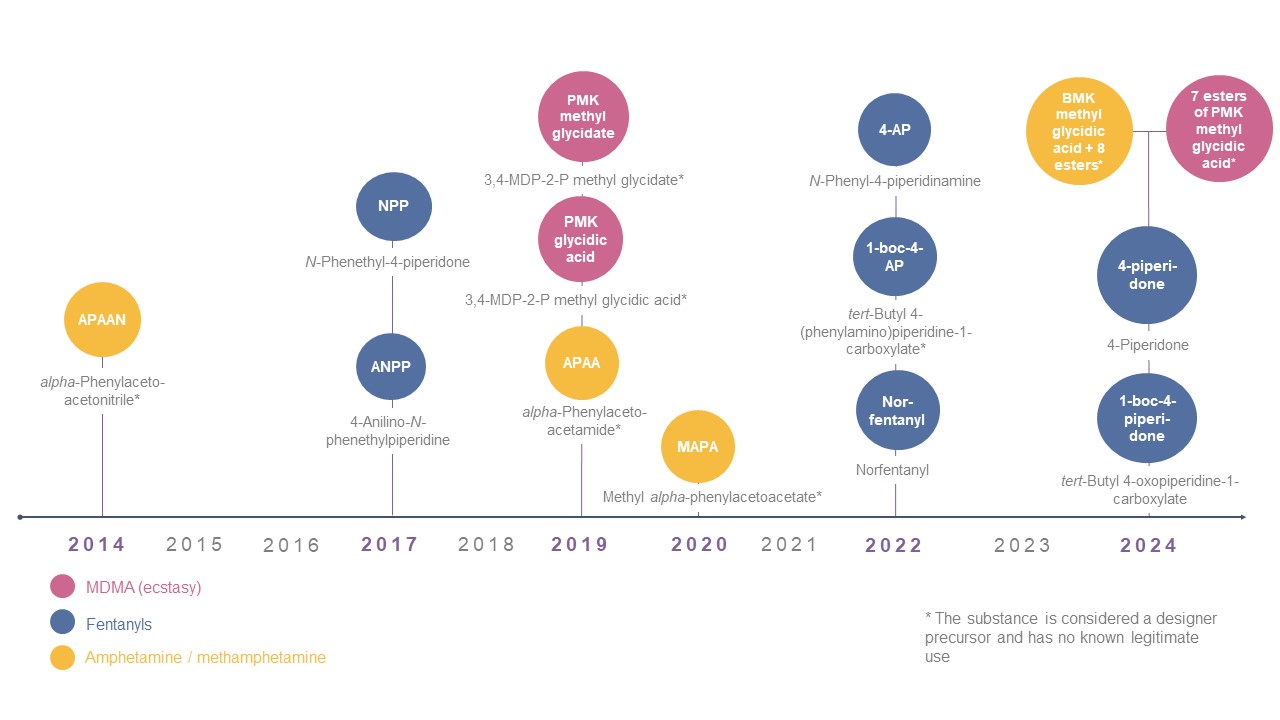
International scheduling of precursors since 2014
Are scheduling decisions at the CND final?
Scheduling decisions are subject to review by the ECOSOC upon the request of any State party. The request for review must be filed within 180 days of receipt of notification of the decision. ECOSOC may confirm, alter or reverse the decision of the CND, and the ECOSOC decision is final.
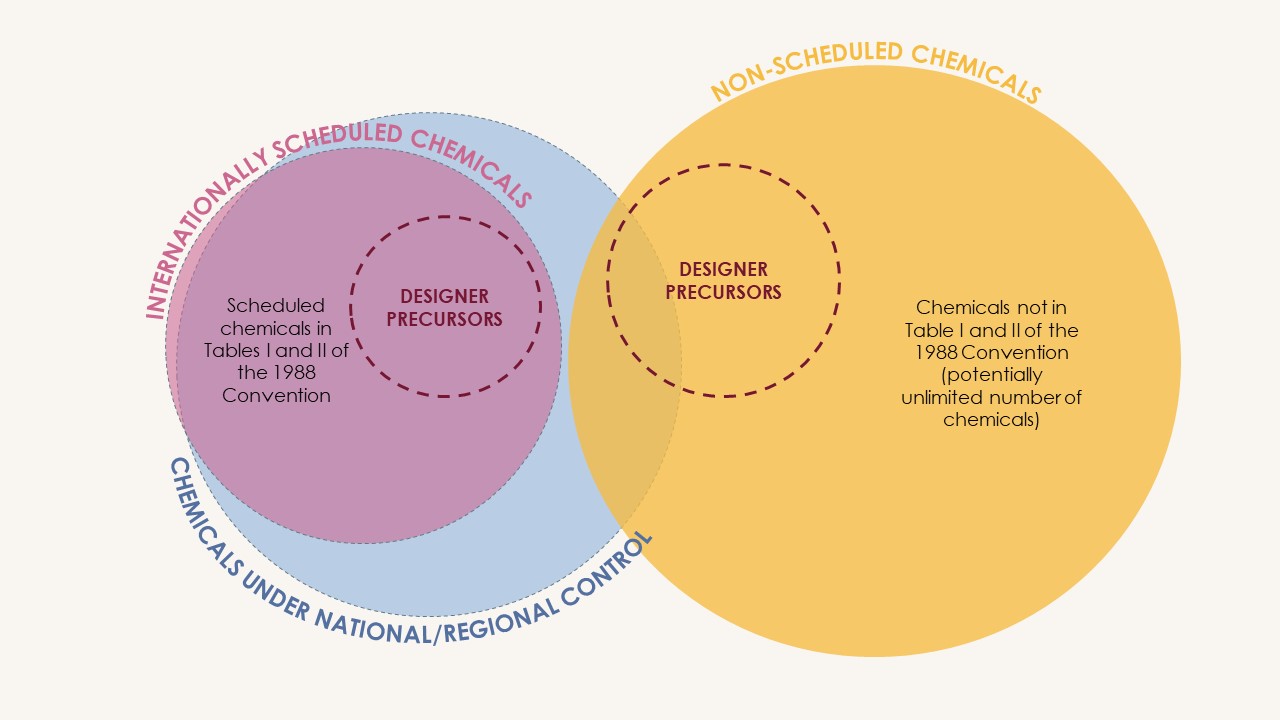
How are closely related chemical substances addressed?
Illicit drug manufacturers often circumvent controls by replacing controlled chemicals with closely related substitutes, including designer precursors
The scheduling is a substance-by-substance process. However, the scope of control of the 1988 Convention is extended to salts and (stereo)isomeric forms of certain scheduled precursors, whenever they exist.
CND resolution 65/3 of March 2022 invited INCB and Governments within their respective purviews to consider derivatives and related chemicals which may readily be converted to or used in place of a to-be-scheduled substance.
Latest news
- International control of two additional fentanyl precursors and two groups of amphetamine-type stimulant precursors enters into force (3 December 2024)
- Decision of the Commission on Narcotics Drugs to follow INCB recommendation to include two fentanyl precursors and 16 precursors of amphetamine-type stimulants in Table I of the 1988 Convention (19 March 2024)
- Statements by Prof. Jallal Toufiq, President of INCB at the Sixty-seventh session of the Commission on Narcotic Drugs on Item 5(a): Changes in the scope of control of substances (19 March 2024)
- INCB takes part in Commission on Narcotic Drugs thematic discussions and reconvened session (8 December 2023)
- International Narcotics Control Board recommends scheduling of 18 precursors of fentanyl and amphetamine-type stimulants (28 November 2023)
Additional information and resources
- 1988 Convention
- Commission on Narcotics Drugs
- UNODC's brochure on scheduling procedures for precursors, narcotic drugs and psychotropic substances
- CND Resolution 65/3 of March 2022